
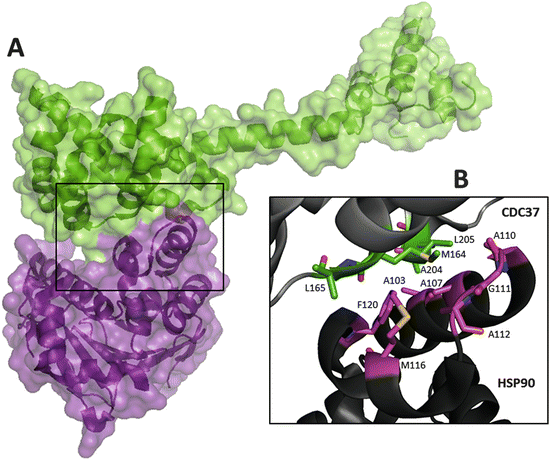
Hsp90 inhibitors can be used as single agents or in combination with other targeted treatments or chemotherapy and radiotherapy. The heat shock protein 90 (Hsp90) is an ATP-dependent molecular chaperone that controls protein maturation and folding, in addition to central regulatory functions of the eukaryotic cell 1. The best known of them, 17-AAG, has shown significant antitumour activity against a broad variety of cancers in preclinical studies, including breast, myeloma, melanoma, prostate and lung cancers. We will review the clinical data on Hsp90 inhibitors in different malignancies. Hsp90 fulfills a housekeeping function in contributing. Hsp90 inhibitors, derived from the natural compound geldanamycin, are attractive targets for anticancer drug development. Hsp90 is an ATP-dependent molecular chaperone that promotes the stabilization of several partially folded client proteins that include steroid hormone receptors, kinases, signal transducers, DNA repair proteins, and structural proteins (Picard, 2002). Heat-shock protein 90 (Hsp90) is an abundant and highly conserved molecular chaperone that is essential for viability in eukaryotes. Inhibition of Hsp90 leads to the degradation of known oncogene products, such as Her2, BRAF and others, leading to the simultaneous blockade of multiple oncogenic transduction pathways. Disruption of Hsp90 leads to client protein degradation and often cell death. Under stressful conditions, Hsp90 stabilizes its client proteins and provides protection to the cell against cellular stressors such as in cancer cells. A growing number of Hsp90 client proteins have been shown to be important for the development, proliferation and survival of several types of cancer. Heat shock protein 90 (Hsp90) is a molecular chaperone involved in the trafficking of proteins in the cell. Hsp90 proteins are the best studied proteins of this family. Heat shock proteins are ubiquitous molecular chaperones involved in posttranslational folding, stability, activation and maturation of many proteins that are essential mediators of signal transduction and cell cycle progression. ABSTRACT The HSP90 molecular chaperone is involved in the activation and cellular stabilization of a range of ‘client’ proteins, of which oncogenic protein kinases and nuclear steroid hormone recep. Heat shock protein 90 (HSP90) molecular chaperones are a family of ubiquitous proteins participating in several cellular functions through the regulation of folding and/or assembly of large multiprotein complexes and client proteins.


 0 kommentar(er)
0 kommentar(er)
